Volume 28, Issue 4 (Autumn 2022)
Intern Med Today 2022, 28(4): 498-513 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hosseininejad-Chafi M, Kianmehr Z, Pooshang Bagheri K, Kazemi-Lomedasht F, Behdani M. Expression of Recombinant Human Programmed Cell Death Protein-1 and Development of Camel Polyclonal Antibody. Intern Med Today 2022; 28 (4) :498-513
URL: http://imtj.gmu.ac.ir/article-1-3928-en.html
URL: http://imtj.gmu.ac.ir/article-1-3928-en.html
Mohammad Hosseininejad-Chafi1 

 , Zahra Kianmehr1
, Zahra Kianmehr1 

 , Kamran Pooshang Bagheri2
, Kamran Pooshang Bagheri2 

 , Fatemeh Kazemi-Lomedasht2
, Fatemeh Kazemi-Lomedasht2 

 , Mahdi Behdani *
, Mahdi Behdani * 

 3
3


 , Zahra Kianmehr1
, Zahra Kianmehr1 

 , Kamran Pooshang Bagheri2
, Kamran Pooshang Bagheri2 

 , Fatemeh Kazemi-Lomedasht2
, Fatemeh Kazemi-Lomedasht2 

 , Mahdi Behdani *
, Mahdi Behdani * 

 3
3
1- Department of Biochemistry, Faculty of Biological Sciences, North Tehran Branch, Islamic Azad University, Tehran, Iran.
2- Department of Medical Biotechnology, Biotechnology Research Center, Laboratory of Venom and Therapeutic Biomolecule, Pasteur Institute of Iran, Tehran, Iran.
3- Department of Medical Biotechnology, Biotechnology Research Center, Laboratory of Venom and Therapeutic Biomolecule, Pasteur Institute of Iran, Tehran, Iran. , behdani@pasteur.ac.ir
2- Department of Medical Biotechnology, Biotechnology Research Center, Laboratory of Venom and Therapeutic Biomolecule, Pasteur Institute of Iran, Tehran, Iran.
3- Department of Medical Biotechnology, Biotechnology Research Center, Laboratory of Venom and Therapeutic Biomolecule, Pasteur Institute of Iran, Tehran, Iran. , behdani@pasteur.ac.ir
Keywords: Programmed cell death protein 1, Heavy chain antibody, Recombinant protein, Polyclonal antibody, Western blotting, ELISA
Full-Text [PDF 5775 kb]
(245 Downloads)
| Abstract (HTML) (537 Views)
Full-Text: (265 Views)
Introduction
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed during activation by T lymphocytes. This protein regulates the function of T cells during various physiological responses, including acute and chronic infection, cancer and autoimmunity, and immune homeostasis [1, 2]. PD-1 is a monomeric surface glycoprotein and is encoded by the PDCD1 gene which consists of 5 exons. This protein has immunoreceptor tyrosine-based inhibitory motifs and an immunoreceptor tyrosine switch motif [3, 4]. PD-1 is inducibly expressed on T lymphocytes with surface markers CD4 and CD8, natural killer (NK) cells, B cells, macrophages, and some subsets of dendritic cells (DC). Some cytokines, such as interleukin-2 (IL-2), interleukin-7 (IL-7), and interferons (IFNs) can induce PD-1 expression in T cells [5]. PD-1 has 2 natural ligands called PD-L1 and PD-L2, which play a role in PD-1: PD-L1/2 signal transmission. PD-1 is mainly expressed on activated T cells, while its main ligand, PD-L1, is expressed on the surface of various cells (including T and B cells, natural killer cells, antigen-presenting cells, epithelial cells, and vascular endothelial cells) [6].
One of the immune checkpoints that many tumors use to escape the immune system is the axis of PD-1 proteins and its ligand. In cancer cells, the PD-L1 gene is overexpressed, and the number of PD-L1 proteins on their surface increases. In cancer, the PD-1/PD-L1 system inhibits T-lymphocyte proliferation, cytokine release, and cytotoxicity [7, 8, 9], which leads to apoptosis of tumor-specific T cells and thus cancer cells. Meanwhile, it provides an opportunity to prevent the immune response to grow and expand [10]. Cancer immunotherapy has recently been developed to design effective treatments to improve the specificity and strength of the immune system against cancer [11]. Allison and Honjo received the 2018 Nobel Prize in Physiology or Medicine for the discovery of cancer treatment by suppressing negative immunomodulation. Their research on programmed immune checkpoints, such as PD-1 and cytotoxic lymphocyte-associated protein 4 (CTLA-4), showed that these proteins have an inhibitory role in immune function and suggested that these immune checkpoints can be inhibited to reactivate T cells to effectively kill cancer cells [12]. This matter was confirmed in numerous subsequent studies and it is currently a proven theory that by inhibiting the function of these inhibitory proteins, therapeutic goals can be achieved in curing cancer.
A large body of evidence suggests that inhibition of PD-1 elicits an effective immune response against cancer cells [13]. Suppression of the PD-1 signaling pathway has shown that the clinical response of patients with various solid tumors and hematologic malignancies relies primarily on T cells to infiltrate the tumor [14]. Furthermore, targeting PD-L1 has been associated with significant clinical response in a wide range of cancer patients. Indeed, PD-1 is a significant immunological marker that plays a negative role in the prognosis of various cancers. Therefore, molecular detection of PD-1 protein is an important goal in many pieces of research [15].
Antibodies are essential everyday reagents in numerous immunotherapy and immunoassay tests. Various animals, such as mice, goats, sheep, horses, rabbits, and camels are used to produce antibodies. Polyclonal antibodies can detect several antigenic indicators, while monoclonal antibodies detect only one epitope and are specific. Antibodies used in diagnostic tests can be obtained from different animal sources, while therapeutic antibodies must be obtained from human sources as they cause immunological reactions in the body. If the therapeutic antibodies are obtained from animals such as mice, their fixed parts should be replaced with the fixed parts of human antibodies to obtain chimeric or humanized antibodies. Camel serum contains two types of antibodies, namely normal antibodies and heavy chain antibodies (HCAB). HCAB is a type of immunoglobulin G (IgG) whose structure consists of only 2 heavy chains, and no light chain. In addition, they have two constant CH2 and CH3 domains, which are similar to the Fc domains of conventional antibodies, but lack the CH1 domain. The antigen binding site in HCAB is unique and has only a one-second variable called VHH, nanoantibody, or nanobody (Nb). The second VHH is small (15-17 kDa) and is known for its high solubility and resistance to temperature, high pressure, and low pH [16-18].
Considering the advantages of camel heavy chain antibodies and the need to produce antibodies against hPD-1, in this study, the gene of the extracellular part of the human PD-1 protein was cloned in bacterial vectors and the recombinant protein was expressed and purified. A camel was then immunized with recombinant PD-1 protein. The efficiency of the obtained polyclonal antibody was evaluated by ELISA and Western blot laboratory methods.
Materials and Methods
Cloning, expression, and purification of human PD-1 protein
The sequence of the extracellular part of human PD-1 (UniprotKB Q15116) containing His-Tag was synthesized and cloned in pGH plasmid and named PD1-pGH. Then, the human PD1 gene was subcloned in the pET-26b vector between NdeI and XhoI sites (Fermentase, USA). To confirm the cloning process, colony-PCR, and confirmatory enzyme digestion methods were used. The final plasmid (PD1-pET26b) was transformed into E. coli BL21-DE3 bacteria and induced PD-1 protein expression with a final concentration of 0. 5 mM isopropyl-beta-di-thiogalactopyranoside (IPTG) for 3 h at 37°C in the incubator. Recombinant protein purification was done using a Ni-NTA affinity chromatography column. Briefly, the sediment of bacteria was dissolved with lysing buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8M Urea, pH 8.0) and sonicated. The cell supernatant was filtered by a 0.45 μm syringe filter and incubated with Ni-NTA resin for 1 h on ice. Then, the resin was poured on the column and washed by washing buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M Urea, pH 6.3), and PD-1 protein was eluted with elution buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M Urea, pH 4.3) and left the column. Protein purity was evaluated by vertical gel electrophoresis (SDS-PAGE). Finally, the protein samples were dialyzed against phosphate buffer solution (PBS) and then the samples were lyophilized.
Western blotting
Western blotting was performed to confirm the identity of the PD-1 protein. The purified protein was transferred to the nitrocellulose membrane after electrophoresis on SDS-PAGE gel. Then, the membrane was incubated with rabbit antibody against His-Tag with a dilution of 1/2000 for 16 h and secondary antibody (anti-rabbit antibody conjugated with HRP (Cell Signaling Technology, USA) for 5 h at 25 degrees. After incubating the membrane with a secondary antibody, 4-chloronaphthal, and hydrogen peroxide (H2O2) substrate were used to observe the protein band. In each step, the nitrocellulose membrane was washed 3 times with PBS buffer.
Immunization of camels
A 9-month-old female Camelus dromedarius were used for immunization. For the first injection, 100 μg of recombinant PD-1 protein was mixed with an equal volume of Freund’s complete adjuvant (Sigma, USA). Then, 6 protein injections with Freund’s incomplete adjuvant subcutaneously, at weekly intervals, were used to increase the immune response. Peripheral blood samples were collected before each injection and sera were separated from whole blood and were used to check the camel immunization process using the ELISA method.
ELISA test
In the ELISA test, 100 μL of a concentration of 1 μg/well of recombinant PD-1 protein was incubated on a 96-well flat microplate with sodium bicarbonate buffer (pH 9.1) for 1 h at 25°C. After that, the plate was washed three times with washing buffer (PBS) and blocked with 4% skim milk. Serum dilutions from 1.200 to 1.14000 were added to the microplate wells and then the microplate was incubated for 1 h at 37 degrees. After washing, rabbit anti-camel antibody and anti-rabbit antibody conjugated with HRP were added to the wells at a dilution of 1/2000 and each was incubated for one hour at room temperature. After the final washing, 100 microliters of TMB solution were added to the wells and the reaction was stopped after 5 min with 2N sulfuric acid solution. Then, the OD of the wells was read at a wavelength of 450 nm.
Verification of gene expression by protein electrophoresis
PD-1 recombinant protein was purified and the quality of the purification was checked by the SDS-PAGE method. For this purpose, 15% acrylamide gel was prepared and the protein samples were boiled with a loading buffer containing 2 ME for 10 min, and the probe was placed next to the marker on the gel, and the electrophoresis process was performed at 80 volts. Then, the gel was stained with Coomassie Blue R250.
Western blot with camel antibody
The purified recombinant PD-1 protein was electrophoresed and then transferred to a nitrocellulose membrane by semi-dry method (45 min, 15V). All washing steps with PBS buffer were performed 3 times in each step. Camel serum containing camel polyclonal antibody against PD-1 recombinant protein with a dilution of 1. 200 was incubated on a nitrocellulose membrane for 1 h at 37 degrees. After washing, anti-camel rabbit antibody and HRP-conjugated anti-rabbit antibody with a dilution of 1/2000 were used for 1 h at room temperature, and 4-chloronaphthal and hydrogen peroxidase (H2O2) substrate were used to observe the protein.
Results
Cloning of human PD-1 protein
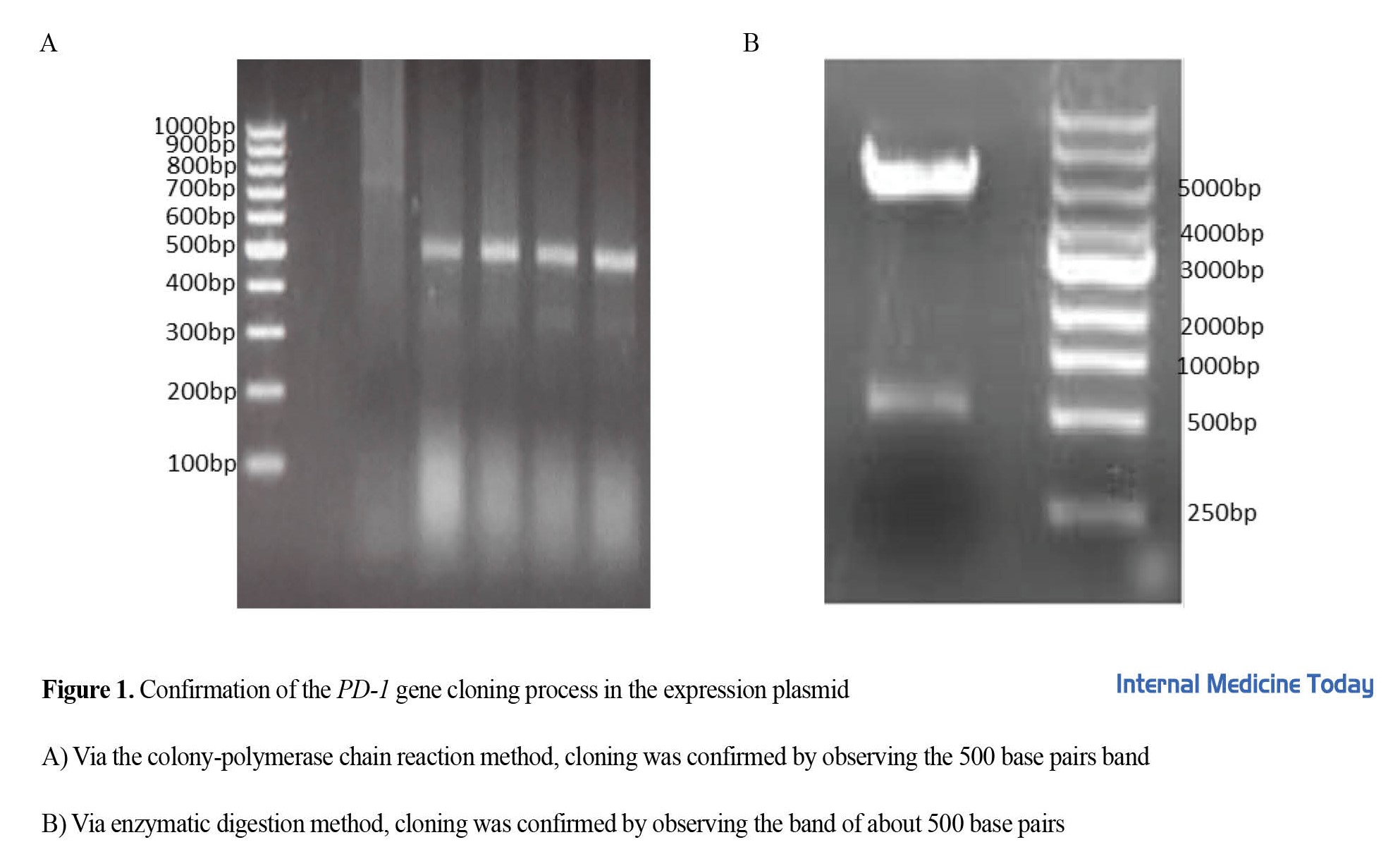
To express and purify the PD-1 protein, the coding sequence of the human PD-1 protein was cloned inside the expression plasmid pET-26 using NdeI and XhoI cloning enzyme sites and transformed into E. coli Top10F’ bacteria. Plasmid cloning and transformation were confirmed by polymerase chain reaction (PCR) (Figure 1A) and plasmid enzymatic digestion (Figure 1B).
Expression and purification of human PD-1 protein
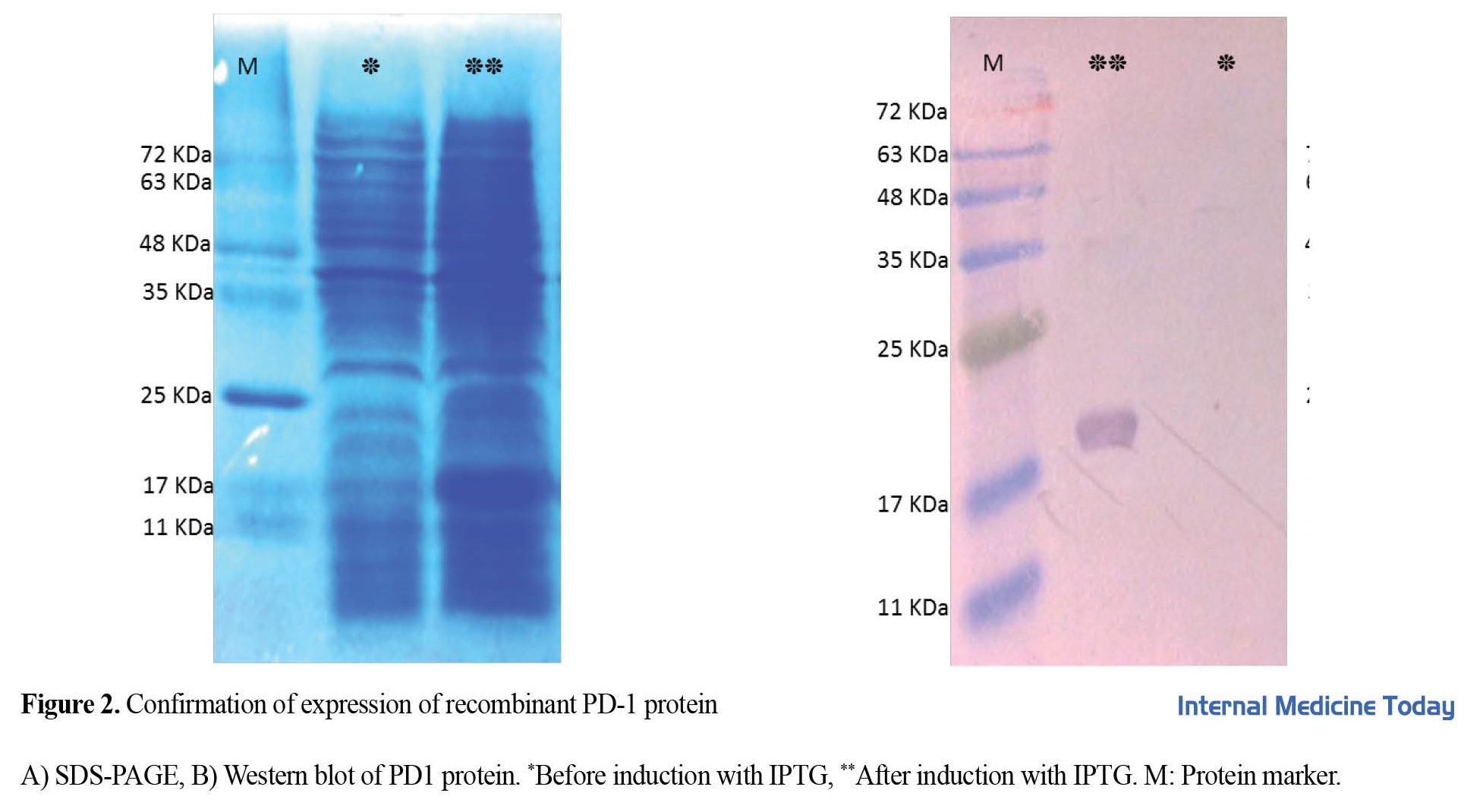
The final recombinant plasmid pET26b-PD1 was transformed into the expression host E. coli BL21-DE3. The gene was induced with 0.5 mM IPTG at 37 degrees for 3 h (volume 100 mL). PD-1 protein expression was confirmed by 15% SDS-PAGE (Figure 2A). Protein purification was done in a denatured state. The purified protein was confirmed by SDS-PAGE and Western blot (Figure 2B and Figure 2C) with anti-His HRP antibody. The yield of protein expression was calculated to be 3.6 mg/L of culture medium.
Checking the immunization of camels
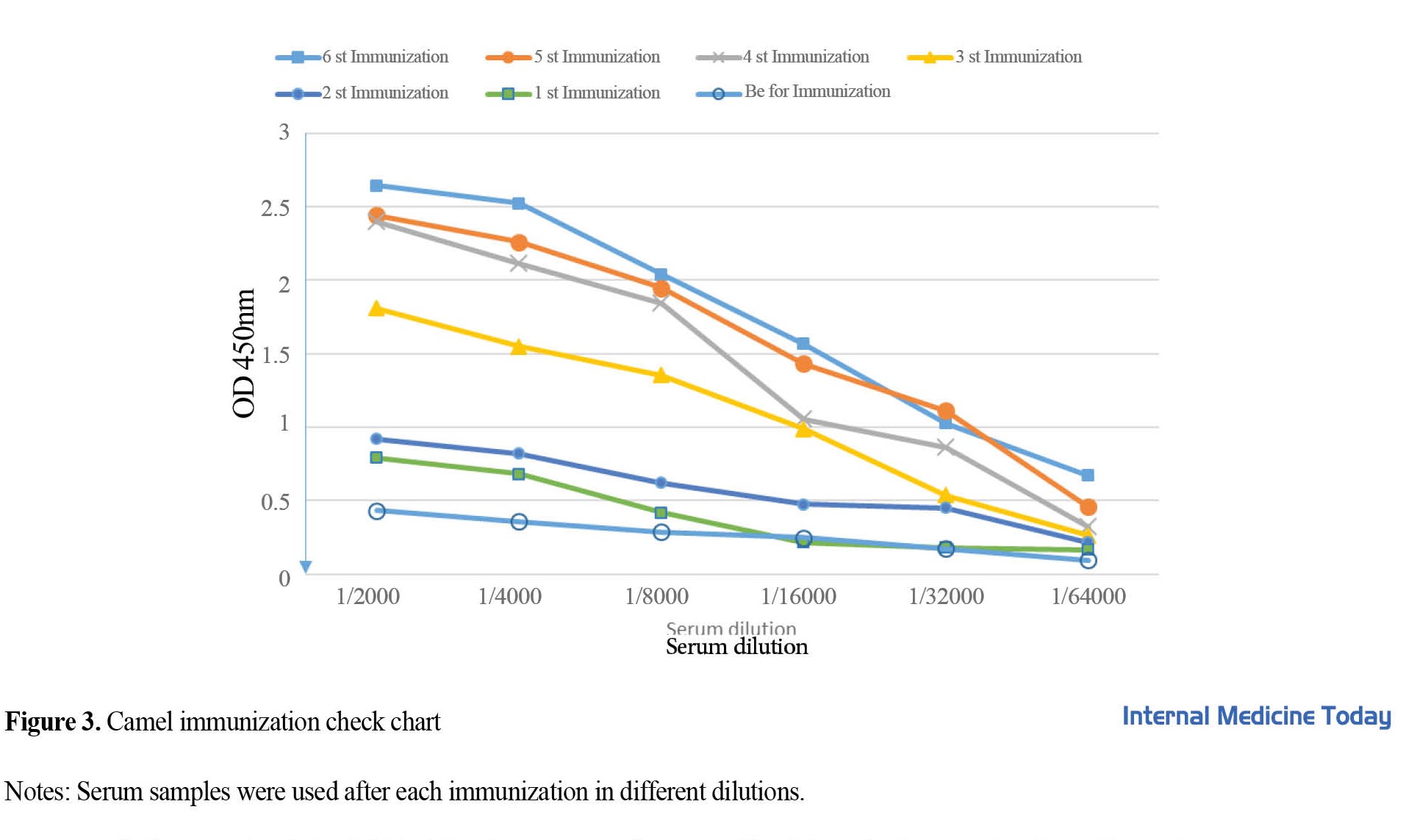
Recombinant PD-1 protein was used to immunize camels. According to the standard protocol, the injections were performed once a week and 6 times. The ELISA method was used to check the humoral immune response in camels. For this purpose, blood was taken from the camel before each injection. As shown in Figure 3, after the third injection, the amount of the specific antibody against the antigen increased. In the third injection, 1/16000 dilution of serum in the ELISA test had OD equal to 1, which indicates a high concentration of the antibody.
Western blot
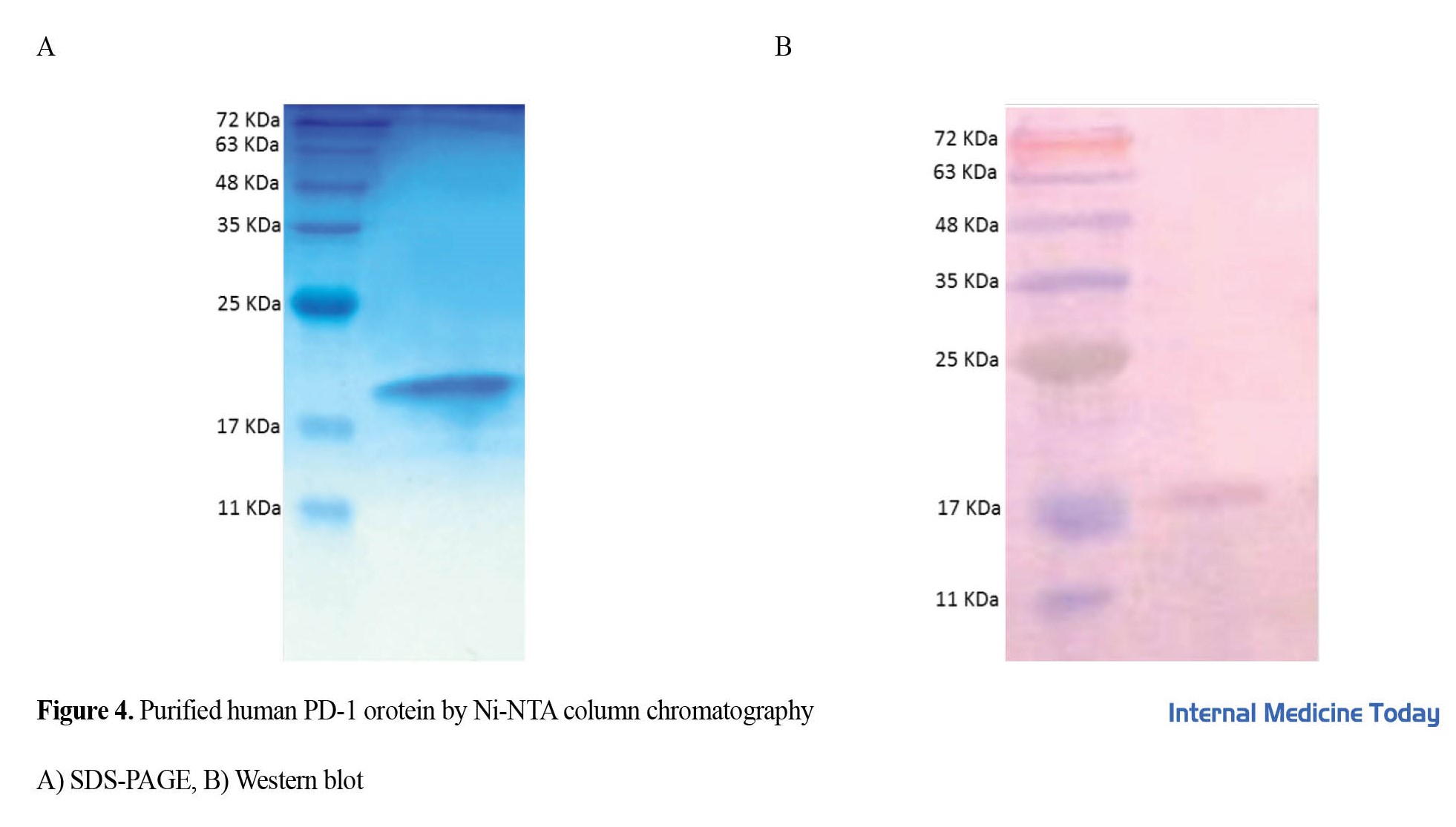
The Western blot method was used to confirm the binding strength of camel serum containing anti-PD-1 polyclonal antibodies to the protein. The binding of polyclonal antibodies to human PD-1 was proved by the Western blotting method (Figure 4), showing that the prepared polyclonal antibody can identify the antigen in the Western blot and can be used for this test. Discussion
PD-1 is an inhibitory receptor expressed on adaptive innate and adaptive immune cells that is critical for regulating inflammation and autoreactivity. Currently, it is known that in different cancers, through the expression of programmed death ligand-1, the activation of PD-1 on immune cells is stopped, thereby cancer cells escape from immune system cells [19]. As a result, inhibitors of these proteins, such as antibodies developed against PD-1 and its ligand can be used to reinvigorate antitumor immunity and improve disease [20]. One of the necessary measures in the treatment using monoclonal antibodies is to measure the amount of the target molecule in the patient being treated. In this context, we can mention 3 diagnostic kits approved by the Food and Drug Administration, which are necessary for the classification of patients and the safe and effective use of the relevant drugs [21]. The Ventana SP142 kit is used to investigate the efficacy of atezolizumab, a monoclonal antibody against PD-L1, based on immunohistochemistry, for diagnosis in patients with bladder carcinoma, triple-negative breast cancer, and non-small cell lung cancer. Dako 28-8 and Dako Omnis kits are also used to check PD-L1 levels in some cancer patients receiving therapeutic antibodies. This shows the importance of examining the expression level of these proteins in patients receiving drugs; therefore, examining PD-1 in laboratory techniques is important.
Despite the diagnostic and therapeutic advantages of monoclonal antibodies, polyclonal antibodies are still used in diagnostic and therapeutic work. Reducing the time and cost of production, having a balanced structure, bearing environmental stress, and the ability to bind to multiple epitopes are some of the advantages that facilitate the production, storage, and application of polyclonal antibodies. Since the discovery of heavy chain antibodies in the serum of camels, this type of antibody has also been widely used in diagnosis and treatment [22].
In this study, the extracellular part of the human PD-1 protein was expressed in the prokaryotic host. Then the human PD-1 protein was purified by Ni-NTA affinity chromatography under denaturing conditions with urea. In a similar study conducted by Zhansaya et al. in 2020, the extracellular part of human PD-1 was cloned in the pET28 vector. In this study, an attempt was made to obtain the protein in a soluble form; therefore, different amounts of IPTG and temperature conditions were evaluated. Finally, the amount of 0.2 mM of IPTG and the temperature of 25 degrees was considered the optimal culture conditions. The protein size in that study was 21 KDa, while in our study it was about 17.5 KDa. The reason for this difference could be related to the place of protein extraction (inside or outside the cell) and the existence of secondary structures in the protein [23]. In the continuation of the study, the purified protein was injected subcutaneously into a 9-month-old camel with 6 repetitions every 2 weeks. The process of camel immunization was investigated by the ELISA method. The data showed that the camel was immunized against the extracellular part of the PD-1 protein with a high titer of camel polyclonal antibody against PD-1. In similar studies conducted by our team regarding camel polyclonal antibodies against [24] LIV-1, [25] CTLA-4, [26] PlGF proteins, it was also shown that recombinant proteins can be used as a suitable source of antigens to stimulate the immune system of camels. Also, in the study of Behdani et al. in 2012, they used VEGFR2 antigen-expressing cells as a source of antigen for camel immunization and showed the effectiveness of the cell as an antigen [27].
It can be concluded that different sources of antigen can be used to immunize camels, similar to other mammals. In the continuation of the study, the binding of camel polyclonal antibody to PD-1 protein was evaluated in the ELISA and Western blot methods, and the results showed that this antibody is capable of binding and identifying human PD-1 protein in the mentioned laboratory tests. This ability has been shown in other subjects as well [24, 26]. One of the applications that camel polyclonal antibodies, given their ability to resist harsh conditions, is to be used as therapeutic antisera for the treatment of patients bitten by poisonous organisms. In the study of Meddeb-Mouelhi et al. in 2003, camel antiserum against scorpion Androctonus australis hector was used and they showed that this antiserum can neutralize the venom [28]. Also, in Behdani et al. ’s study, the neutralizing ability of camel antiserum in neutralizing the scorpion Hemiscorpius lepturus was shown [29]. In the continuation of this study, polyclonal antibodies against PD-1 can be evaluated as a therapeutic polyclonal antiserum to prevent the growth of cancer cells in vitro and in vivo.
Conclusion
Finally, the resulting polyclonal antibody can be used as a suitable antibody for diagnostic tests such as ELISA, western blot, flow cytometry and immunohistochemistry.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of the Pasteur Institute of Iran approved this article (Code: IR.PII.REC.1400.041).
Funding
This article was done with the financial support of the Pasteur Institute of Iran (Project No.: 1805).
Authors' contributions
The authors contributed equally to preparing this paper.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
We are grateful for the cooperation of Delawar Shahbazada for his effective efforts in achieving the results of this project.
References
Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed during activation by T lymphocytes. This protein regulates the function of T cells during various physiological responses, including acute and chronic infection, cancer and autoimmunity, and immune homeostasis [1, 2]. PD-1 is a monomeric surface glycoprotein and is encoded by the PDCD1 gene which consists of 5 exons. This protein has immunoreceptor tyrosine-based inhibitory motifs and an immunoreceptor tyrosine switch motif [3, 4]. PD-1 is inducibly expressed on T lymphocytes with surface markers CD4 and CD8, natural killer (NK) cells, B cells, macrophages, and some subsets of dendritic cells (DC). Some cytokines, such as interleukin-2 (IL-2), interleukin-7 (IL-7), and interferons (IFNs) can induce PD-1 expression in T cells [5]. PD-1 has 2 natural ligands called PD-L1 and PD-L2, which play a role in PD-1: PD-L1/2 signal transmission. PD-1 is mainly expressed on activated T cells, while its main ligand, PD-L1, is expressed on the surface of various cells (including T and B cells, natural killer cells, antigen-presenting cells, epithelial cells, and vascular endothelial cells) [6].
One of the immune checkpoints that many tumors use to escape the immune system is the axis of PD-1 proteins and its ligand. In cancer cells, the PD-L1 gene is overexpressed, and the number of PD-L1 proteins on their surface increases. In cancer, the PD-1/PD-L1 system inhibits T-lymphocyte proliferation, cytokine release, and cytotoxicity [7, 8, 9], which leads to apoptosis of tumor-specific T cells and thus cancer cells. Meanwhile, it provides an opportunity to prevent the immune response to grow and expand [10]. Cancer immunotherapy has recently been developed to design effective treatments to improve the specificity and strength of the immune system against cancer [11]. Allison and Honjo received the 2018 Nobel Prize in Physiology or Medicine for the discovery of cancer treatment by suppressing negative immunomodulation. Their research on programmed immune checkpoints, such as PD-1 and cytotoxic lymphocyte-associated protein 4 (CTLA-4), showed that these proteins have an inhibitory role in immune function and suggested that these immune checkpoints can be inhibited to reactivate T cells to effectively kill cancer cells [12]. This matter was confirmed in numerous subsequent studies and it is currently a proven theory that by inhibiting the function of these inhibitory proteins, therapeutic goals can be achieved in curing cancer.
A large body of evidence suggests that inhibition of PD-1 elicits an effective immune response against cancer cells [13]. Suppression of the PD-1 signaling pathway has shown that the clinical response of patients with various solid tumors and hematologic malignancies relies primarily on T cells to infiltrate the tumor [14]. Furthermore, targeting PD-L1 has been associated with significant clinical response in a wide range of cancer patients. Indeed, PD-1 is a significant immunological marker that plays a negative role in the prognosis of various cancers. Therefore, molecular detection of PD-1 protein is an important goal in many pieces of research [15].
Antibodies are essential everyday reagents in numerous immunotherapy and immunoassay tests. Various animals, such as mice, goats, sheep, horses, rabbits, and camels are used to produce antibodies. Polyclonal antibodies can detect several antigenic indicators, while monoclonal antibodies detect only one epitope and are specific. Antibodies used in diagnostic tests can be obtained from different animal sources, while therapeutic antibodies must be obtained from human sources as they cause immunological reactions in the body. If the therapeutic antibodies are obtained from animals such as mice, their fixed parts should be replaced with the fixed parts of human antibodies to obtain chimeric or humanized antibodies. Camel serum contains two types of antibodies, namely normal antibodies and heavy chain antibodies (HCAB). HCAB is a type of immunoglobulin G (IgG) whose structure consists of only 2 heavy chains, and no light chain. In addition, they have two constant CH2 and CH3 domains, which are similar to the Fc domains of conventional antibodies, but lack the CH1 domain. The antigen binding site in HCAB is unique and has only a one-second variable called VHH, nanoantibody, or nanobody (Nb). The second VHH is small (15-17 kDa) and is known for its high solubility and resistance to temperature, high pressure, and low pH [16-18].
Considering the advantages of camel heavy chain antibodies and the need to produce antibodies against hPD-1, in this study, the gene of the extracellular part of the human PD-1 protein was cloned in bacterial vectors and the recombinant protein was expressed and purified. A camel was then immunized with recombinant PD-1 protein. The efficiency of the obtained polyclonal antibody was evaluated by ELISA and Western blot laboratory methods.
Materials and Methods
Cloning, expression, and purification of human PD-1 protein
The sequence of the extracellular part of human PD-1 (UniprotKB Q15116) containing His-Tag was synthesized and cloned in pGH plasmid and named PD1-pGH. Then, the human PD1 gene was subcloned in the pET-26b vector between NdeI and XhoI sites (Fermentase, USA). To confirm the cloning process, colony-PCR, and confirmatory enzyme digestion methods were used. The final plasmid (PD1-pET26b) was transformed into E. coli BL21-DE3 bacteria and induced PD-1 protein expression with a final concentration of 0. 5 mM isopropyl-beta-di-thiogalactopyranoside (IPTG) for 3 h at 37°C in the incubator. Recombinant protein purification was done using a Ni-NTA affinity chromatography column. Briefly, the sediment of bacteria was dissolved with lysing buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8M Urea, pH 8.0) and sonicated. The cell supernatant was filtered by a 0.45 μm syringe filter and incubated with Ni-NTA resin for 1 h on ice. Then, the resin was poured on the column and washed by washing buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M Urea, pH 6.3), and PD-1 protein was eluted with elution buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M Urea, pH 4.3) and left the column. Protein purity was evaluated by vertical gel electrophoresis (SDS-PAGE). Finally, the protein samples were dialyzed against phosphate buffer solution (PBS) and then the samples were lyophilized.
Western blotting
Western blotting was performed to confirm the identity of the PD-1 protein. The purified protein was transferred to the nitrocellulose membrane after electrophoresis on SDS-PAGE gel. Then, the membrane was incubated with rabbit antibody against His-Tag with a dilution of 1/2000 for 16 h and secondary antibody (anti-rabbit antibody conjugated with HRP (Cell Signaling Technology, USA) for 5 h at 25 degrees. After incubating the membrane with a secondary antibody, 4-chloronaphthal, and hydrogen peroxide (H2O2) substrate were used to observe the protein band. In each step, the nitrocellulose membrane was washed 3 times with PBS buffer.
Immunization of camels
A 9-month-old female Camelus dromedarius were used for immunization. For the first injection, 100 μg of recombinant PD-1 protein was mixed with an equal volume of Freund’s complete adjuvant (Sigma, USA). Then, 6 protein injections with Freund’s incomplete adjuvant subcutaneously, at weekly intervals, were used to increase the immune response. Peripheral blood samples were collected before each injection and sera were separated from whole blood and were used to check the camel immunization process using the ELISA method.
ELISA test
In the ELISA test, 100 μL of a concentration of 1 μg/well of recombinant PD-1 protein was incubated on a 96-well flat microplate with sodium bicarbonate buffer (pH 9.1) for 1 h at 25°C. After that, the plate was washed three times with washing buffer (PBS) and blocked with 4% skim milk. Serum dilutions from 1.200 to 1.14000 were added to the microplate wells and then the microplate was incubated for 1 h at 37 degrees. After washing, rabbit anti-camel antibody and anti-rabbit antibody conjugated with HRP were added to the wells at a dilution of 1/2000 and each was incubated for one hour at room temperature. After the final washing, 100 microliters of TMB solution were added to the wells and the reaction was stopped after 5 min with 2N sulfuric acid solution. Then, the OD of the wells was read at a wavelength of 450 nm.
Verification of gene expression by protein electrophoresis
PD-1 recombinant protein was purified and the quality of the purification was checked by the SDS-PAGE method. For this purpose, 15% acrylamide gel was prepared and the protein samples were boiled with a loading buffer containing 2 ME for 10 min, and the probe was placed next to the marker on the gel, and the electrophoresis process was performed at 80 volts. Then, the gel was stained with Coomassie Blue R250.
Western blot with camel antibody
The purified recombinant PD-1 protein was electrophoresed and then transferred to a nitrocellulose membrane by semi-dry method (45 min, 15V). All washing steps with PBS buffer were performed 3 times in each step. Camel serum containing camel polyclonal antibody against PD-1 recombinant protein with a dilution of 1. 200 was incubated on a nitrocellulose membrane for 1 h at 37 degrees. After washing, anti-camel rabbit antibody and HRP-conjugated anti-rabbit antibody with a dilution of 1/2000 were used for 1 h at room temperature, and 4-chloronaphthal and hydrogen peroxidase (H2O2) substrate were used to observe the protein.
Results
Cloning of human PD-1 protein
To express and purify the PD-1 protein, the coding sequence of the human PD-1 protein was cloned inside the expression plasmid pET-26 using NdeI and XhoI cloning enzyme sites and transformed into E. coli Top10F’ bacteria. Plasmid cloning and transformation were confirmed by polymerase chain reaction (PCR) (Figure 1A) and plasmid enzymatic digestion (Figure 1B).
Expression and purification of human PD-1 protein
The final recombinant plasmid pET26b-PD1 was transformed into the expression host E. coli BL21-DE3. The gene was induced with 0.5 mM IPTG at 37 degrees for 3 h (volume 100 mL). PD-1 protein expression was confirmed by 15% SDS-PAGE (Figure 2A). Protein purification was done in a denatured state. The purified protein was confirmed by SDS-PAGE and Western blot (Figure 2B and Figure 2C) with anti-His HRP antibody. The yield of protein expression was calculated to be 3.6 mg/L of culture medium.
Checking the immunization of camels
Recombinant PD-1 protein was used to immunize camels. According to the standard protocol, the injections were performed once a week and 6 times. The ELISA method was used to check the humoral immune response in camels. For this purpose, blood was taken from the camel before each injection. As shown in Figure 3, after the third injection, the amount of the specific antibody against the antigen increased. In the third injection, 1/16000 dilution of serum in the ELISA test had OD equal to 1, which indicates a high concentration of the antibody.
Western blot
The Western blot method was used to confirm the binding strength of camel serum containing anti-PD-1 polyclonal antibodies to the protein. The binding of polyclonal antibodies to human PD-1 was proved by the Western blotting method (Figure 4), showing that the prepared polyclonal antibody can identify the antigen in the Western blot and can be used for this test. Discussion
PD-1 is an inhibitory receptor expressed on adaptive innate and adaptive immune cells that is critical for regulating inflammation and autoreactivity. Currently, it is known that in different cancers, through the expression of programmed death ligand-1, the activation of PD-1 on immune cells is stopped, thereby cancer cells escape from immune system cells [19]. As a result, inhibitors of these proteins, such as antibodies developed against PD-1 and its ligand can be used to reinvigorate antitumor immunity and improve disease [20]. One of the necessary measures in the treatment using monoclonal antibodies is to measure the amount of the target molecule in the patient being treated. In this context, we can mention 3 diagnostic kits approved by the Food and Drug Administration, which are necessary for the classification of patients and the safe and effective use of the relevant drugs [21]. The Ventana SP142 kit is used to investigate the efficacy of atezolizumab, a monoclonal antibody against PD-L1, based on immunohistochemistry, for diagnosis in patients with bladder carcinoma, triple-negative breast cancer, and non-small cell lung cancer. Dako 28-8 and Dako Omnis kits are also used to check PD-L1 levels in some cancer patients receiving therapeutic antibodies. This shows the importance of examining the expression level of these proteins in patients receiving drugs; therefore, examining PD-1 in laboratory techniques is important.
Despite the diagnostic and therapeutic advantages of monoclonal antibodies, polyclonal antibodies are still used in diagnostic and therapeutic work. Reducing the time and cost of production, having a balanced structure, bearing environmental stress, and the ability to bind to multiple epitopes are some of the advantages that facilitate the production, storage, and application of polyclonal antibodies. Since the discovery of heavy chain antibodies in the serum of camels, this type of antibody has also been widely used in diagnosis and treatment [22].
In this study, the extracellular part of the human PD-1 protein was expressed in the prokaryotic host. Then the human PD-1 protein was purified by Ni-NTA affinity chromatography under denaturing conditions with urea. In a similar study conducted by Zhansaya et al. in 2020, the extracellular part of human PD-1 was cloned in the pET28 vector. In this study, an attempt was made to obtain the protein in a soluble form; therefore, different amounts of IPTG and temperature conditions were evaluated. Finally, the amount of 0.2 mM of IPTG and the temperature of 25 degrees was considered the optimal culture conditions. The protein size in that study was 21 KDa, while in our study it was about 17.5 KDa. The reason for this difference could be related to the place of protein extraction (inside or outside the cell) and the existence of secondary structures in the protein [23]. In the continuation of the study, the purified protein was injected subcutaneously into a 9-month-old camel with 6 repetitions every 2 weeks. The process of camel immunization was investigated by the ELISA method. The data showed that the camel was immunized against the extracellular part of the PD-1 protein with a high titer of camel polyclonal antibody against PD-1. In similar studies conducted by our team regarding camel polyclonal antibodies against [24] LIV-1, [25] CTLA-4, [26] PlGF proteins, it was also shown that recombinant proteins can be used as a suitable source of antigens to stimulate the immune system of camels. Also, in the study of Behdani et al. in 2012, they used VEGFR2 antigen-expressing cells as a source of antigen for camel immunization and showed the effectiveness of the cell as an antigen [27].
It can be concluded that different sources of antigen can be used to immunize camels, similar to other mammals. In the continuation of the study, the binding of camel polyclonal antibody to PD-1 protein was evaluated in the ELISA and Western blot methods, and the results showed that this antibody is capable of binding and identifying human PD-1 protein in the mentioned laboratory tests. This ability has been shown in other subjects as well [24, 26]. One of the applications that camel polyclonal antibodies, given their ability to resist harsh conditions, is to be used as therapeutic antisera for the treatment of patients bitten by poisonous organisms. In the study of Meddeb-Mouelhi et al. in 2003, camel antiserum against scorpion Androctonus australis hector was used and they showed that this antiserum can neutralize the venom [28]. Also, in Behdani et al. ’s study, the neutralizing ability of camel antiserum in neutralizing the scorpion Hemiscorpius lepturus was shown [29]. In the continuation of this study, polyclonal antibodies against PD-1 can be evaluated as a therapeutic polyclonal antiserum to prevent the growth of cancer cells in vitro and in vivo.
Conclusion
Finally, the resulting polyclonal antibody can be used as a suitable antibody for diagnostic tests such as ELISA, western blot, flow cytometry and immunohistochemistry.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of the Pasteur Institute of Iran approved this article (Code: IR.PII.REC.1400.041).
Funding
This article was done with the financial support of the Pasteur Institute of Iran (Project No.: 1805).
Authors' contributions
The authors contributed equally to preparing this paper.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
We are grateful for the cooperation of Delawar Shahbazada for his effective efforts in achieving the results of this project.
References
- Bardhan K, Anagnostou T, Boussiotis VA. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Frontiers in Immunology. 2016; 7: 550. [DOI: 10. 3389/fimmu. 2016. 00550] [PMID] [PMCID]
- Shrimali RK, Janik JE, Abu-Eid R, Mkrtichyan M, Khleif SN. Programmed death-1 & its ligands: Promising targets for cancer immunotherapy. Immunotherapy. 2015; 7(7): 777-92. [DOI: 10. 2217/imt. 15. 49] [PMID]
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO Journal. 1992; 11(11): 3887-95. [DOI: 10. 1002/j. 1460-2075. 1992. tb05481. x] [PMID] [PMCID]
- Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1). Genomics. 1994; 23(3): 704-6. [DOI: 10. 1006/geno. 1994. 1562] [PMID]
- Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. Journal of Immunology. 2011; 186(5): 2772-9. [DOI: 10. 4049/jimmunol. 1003208] [PMID]
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. International Immunology. 2007; 19(7): 813-24. [DOI: 10. 1093/intimm/dxm057] [PMID]
- Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Research and Treatment. 2013; 139(3): 667-76. [DOI: 10. 1007/s10549-013-2581-3] [PMID] [PMCID]
- Dong Y, Wong JSL, Sugimura R, Lam KO, Li B, Kwok GGW, et al. Recent advances and future prospects in Immune Checkpoint (ICI)-based combination therapy for advanced HCC. Cancers (Basel). 2021; 13(8): 1949. [DOI: 10. 3390/cancers13081949] [PMID] [PMCID]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015; 348(6230): 56-61. [DOI: 10. 1126/science. aaa8172] [PMID]
- Sun S, Fei X, Mao Y, Wang X, Garfield DH, Huang O, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunology, Immunotherapy: CII. 2014; 63(4): 395-406. [DOI: 10. 1007/s00262-014-1519-x] [PMID]
- Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, et al. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. Journal of Cellular Physiology. 2019; 234(10): 16824-37. [DOI: 10. 1002/jcp. 28358] [PMID]
- Ljunggren HG, Jonsson R, Hoglund P. Seminal immunologic discoveries with direct clinical implications: The 2018 Nobel Prize in Physiology or Medicine honours discoveries in cancer immunotherapy. Scandinavian Journal of Immunology. 2018; 88(6): e12731. [DOI: 10. 1111/sji. 12731] [PMID]
- Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with Anti-OX40. Clinical Cancer Research. 2017; 23(20): 6165-77. [DOI: 10. 1158/1078-0432. CCR-16-2677] [PMID] [PMCID]
- Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. Journal of Biomedical Science. 2017; 24(1): 26. [DOI: 10. 1186/s12929-017-0329-9] [PMID] [PMCID]
- Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: A review. JAMA Oncology. 2016; 2(9): 1217-22. [DOI: 10. 1001/jamaoncol. 2016. 0639] [PMID]
- Muyldermans S. Applications of nanobodies. Annual Review of Animal Biosciences. 2021; 9: 401-21. [DOI: 10. 1146/annurev-animal-021419-083831] [PMID]
- Muyldermans S. A guide to: Generation and design of nanobodies. The FEBS Journal. 2021; 288(7): 2084-102. [DOI: 10. 1111/febs. 15515] [PMID] [PMCID]
- Rahbarizadeh F, Ahmadvand D, Sharifzadeh Z. Nanobody; an old concept and new vehicle for immunotargeting. Immunological Investigations. 2011; 40(3): 299-338. [DOI: 10. 3109/08820139. 2010. 542228] [PMID]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nature Medicine. 2002; 8(8): 793-800. [DOI: 10. 1038/nm730] [PMID]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine. 2012; 366(26): 2443-54. [DOI: 10. 1056/NEJMoa1200690] [PMID] [PMCID]
- Food and Drug Administration. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). Maryland: Food and Drug Administration; 2022. [Link]
- Pillay TS, Muyldermans S. Application of single-domain antibodies (“Nanobodies”) to laboratory diagnosis. Annals of Laboratory Medicine. 2021; 41(6): 549-58. [DOI: 10. 3343/alm. 2021. 41. 6. 549] [PMID] [PMCID]
- Zhansaya A, Kanatbek M, Kanat T, Bakhytkali I, Darkhan K, Arman K, et al. Recombinant Expression and Purification of Extracellular Domain of the Programmed Cell Death Protein Receptor. Reports of Biochemistry & Molecular Biology. 2020; 8(4): 347-57. [PMID]
- Ghaderi H, Noormohammadi Z, Habibi-Anbouhi M, Kazemi-Lomedasht F, Behdani M. [Preparation of heavy chain polyclonal antibody against zinc transporter SLC39A6 and its diagnostic application (Persian)]. Tehran University Medical Journal. 2021; 79(4): 274-80. [Link]
- Sotoudeh N, Noormohammadi Z, Habibi-Anbouhi M, Kazemi-Lomedasht F, Behdani M. Evaluation of laboratory application of camelid sera containing heavy-chain polyclonal antibody against recombinant cytotoxic T-lymphocyte-associated protein-4. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy. 2019; 38(6): 235-41. [DOI: 10. 1089/mab. 2019. 0031] [PMID]
- Arezumand R, Mahdian R, Behdani M, Khanahmad H, Langari J, Namvarasl N, et al. Recombinant expression and purification of human placental growth factor 1 and specific camel heavy chain polyclonal antibody preparation. Saudi Journal of Biological Sciences. 2014; 21(1): 35-9. [DOI: 10. 1016/j. sjbs. 2013. 04. 008] [PMID] [PMCID]
- Behdani M, Zeinali S, Khanahmad H, Karimipour M, Asadzadeh N, Azadmanesh K, et al. Generation and characterization of a functional Nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Molecular Immunology. 2012; 50(1-2): 35-41. [DOI: 10. 1016/j. molimm. 2011. 11. 013] [PMID]
- Meddeb-Mouelhi F, Bouhaouala-Zahar B, Benlasfar Z, Hammadi M, Mejri T, Moslah M, et al. Immunized camel sera and derived immunoglobulin subclasses neutralizing Androctonus australis hector scorpion toxins. Toxicon. 2003; 42(7):785-91. [DOI: 10. 1016/j. toxicon. 2003. 10. 021] [PMID]
- Behdani M, Hosseini Nejad Chafi M, Zeinali S, Karimipour M, Khanahmad-Shahreza H, Ghasemi P, et al. [Antiserum production in immunized camel by the venom of Hemiscorpius lepturus scorpion: Evaluation of neutralizing test in vivo (Persian)]. Tehran University Medical Journal. 2010; 68(5):268-73. [Link]
Type of Study: Original |
Subject:
Basic Medical Science
Received: 2022/08/31 | Accepted: 2022/09/23 | Published: 2022/09/23
Received: 2022/08/31 | Accepted: 2022/09/23 | Published: 2022/09/23
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |